[ad_1]

Did you know that the discovery of helium, an element we associate with balloons, is also linked to India? Here, an artist floats in the air with supporters attached to helium balloons. | Photo Credit: AFP
After all, what does a gas that we usually associate with balloons have to do with the city of Guntur in Andhra Pradesh? Apparently, everything! However, to be completely clear, it is important to point out that it all started with the observation of the Sun from Guntur, before the existence of helium was discovered on Earth.

A sketch of the solar eclipse of 1868. | Photo Credit: Wikimedia Commons
Helium, which makes up about a quarter of all matter in the universe, is the second most abundant element in the universe after hydrogen. Despite this, helium is rare on Earth, only being released as a byproduct when heavier elements undergo radioactive decay. Unless it is produced deep underground or trapped within rocks, the ultra-light non-reactive helium usually flies away and disappears into space.
spans decades
The story of the discovery of helium is a long and elaborate one that spans the better part of a century. Though the Guntur episode is an important one, it falls somewhere in the middle of the whole story. To begin with, we have to go back more than two centuries to 1814.
Much information about a substance and its composition can be obtained by studying the light it absorbs or emits. With its ability to spread light into measurable wavelengths, spectroscopes were to change the way scientists studied the chemical composition of almost everything.
Fraunhofer Lines
Using an early version of the spectroscope, German optical lens maker and physicist Joseph von Fraunhofer created a spectrum that was so broad that deep black lines that interrupted the normal colors could be seen. Although he did not understand what they were, they now bear his name (Fraunhofer lines) and this marked a new beginning in the study of spectral lines to better understand substances.
By 1859, Germans Gustav Kirchhoff and Robert Bunsen had mastered the art of using the analysis of light to infer the chemical composition of the sun and stars. The physician-chemist duo, credited with inventing the modern spectroscope as we know it today, discovered that elements in the spectroscope produced bright lines of light when heated, and that these lines sometimes corresponded to Fraunhofer lines.
Journey to India
As the popular belief at that time was that the spectrum of the Sun could be seen only during an eclipse, astronomers were eagerly waiting for the total eclipse of 1868. Since the duration of the eclipse was about six minutes – a long time in the context – there was ample time for observation. The path of totality passed through every corner of India, forcing the scientific community from all over the world to turn to India.
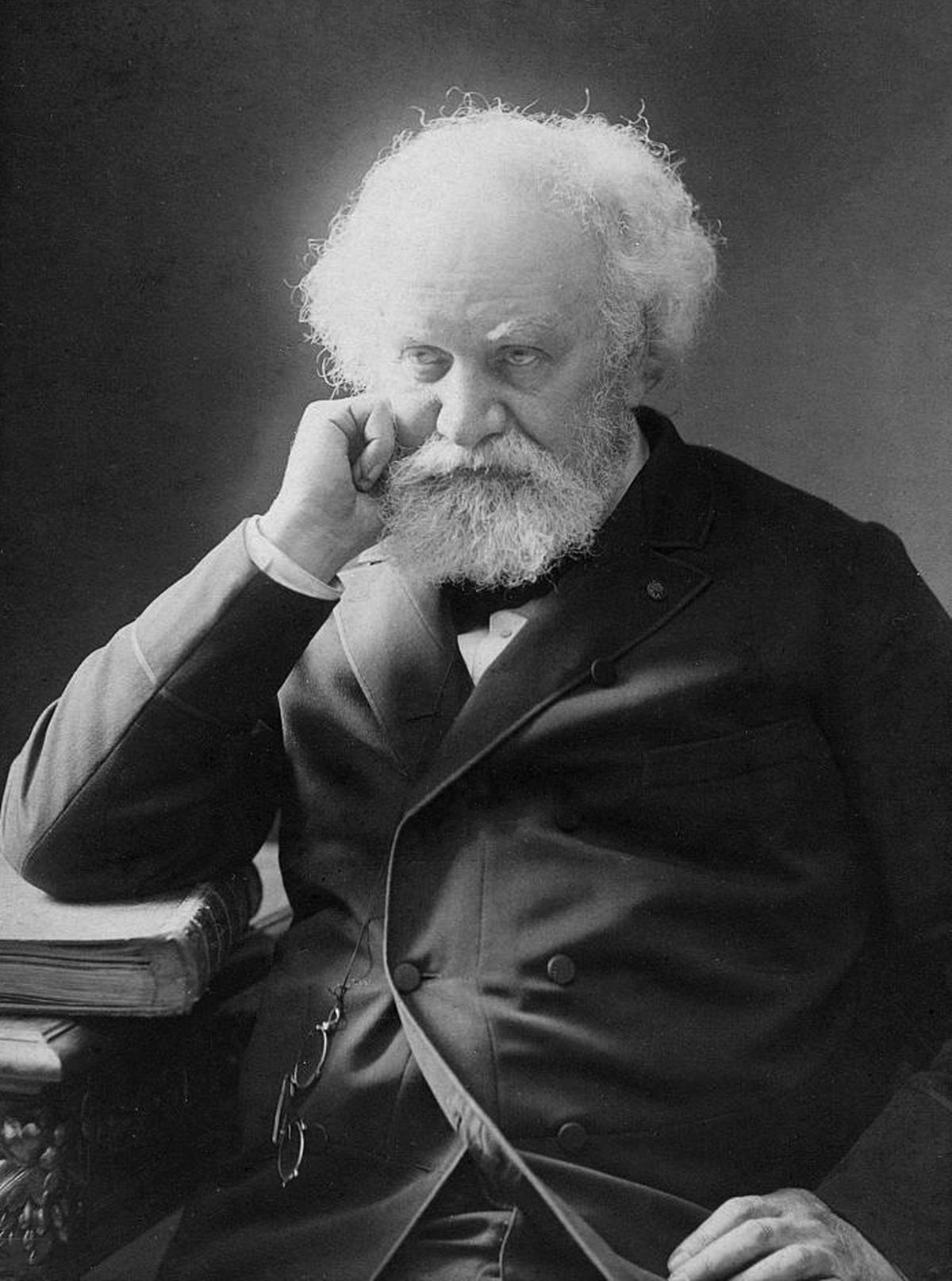
French astronomer Pierre Jules Cesar Janssen. | Photo Credit: Wikimedia Commons
Among them was French astronomer Pierre Jules Cesar Janssen, a scholar who pursued eclipses and had already made a name for himself in the field of solar spectrum. While British astronomer and director of Madras Observatory Norman Pogson headed to Machilipatnam (now a town in Andhra Pradesh), Janssen and another British team headed to Guntur. Apart from the coastal nature of Machilipatnam and hence the risk of fog and cloud, Janssen’s decision to choose Guntur may have been linked to the fact that the place was under French rule and was still full of French traders.
An unusual spectral line
The eclipse took place on August 18, 1868 and was well observed by various teams stationed in different parts of India and elsewhere. A few days later, Pogson’s observation of something unusual – a spectral line close to sodium, but not exactly the same – reached astronomical circles.
Janssen correctly predicted that the line probably belonged to an element never seen on Earth before. He also realized that, using a modified spectroscope, it should be possible to observe the Sun’s spectral lines any day, without having to wait for an eclipse to occur.
in harmony with each other
At about the same time that Janssen was taking these giant steps toward the discovery of helium, Joseph Norman Lockyer, a London-based civil servant and amateur astronomer with a great interest in the Sun, was independently arriving at the same results. Either on the same day, or within a few days of each other in October, the French Academy of Sciences received scientific papers from both men revealing their work that would allow the observation of solar prominences without eclipses, and possibly the possibility of observing a new element.

Sir Joseph Norman Lockyer. | Photo credit: Wellcome Library, London. Wellcome Images / Wikimedia Commons
Rather than trying to decide whether one of them should be given the credit, it was decided that the two men would share the merit of their findings, each’s work confirming that of the other. However, this did not mean that he had won the confidence of the entire scientific community and he faced criticism for his suggestion that the spectral line might be related to an exotic element.
Ramsay’s discovery
Even though Lockyer named the element helium, it took more than 25 years for the element to be “discovered” on Earth. The credit goes to Scottish chemist William Ramsay, who, while working with uranium ore in 1895, not only isolated helium but also showed that it was a product of the radioactive decay of radium.
In the years since its discovery, helium has been put to use serving many important purposes. Yes, helium gas can be used to fill balloons that are more versatile and durable than air-filled balloons. The element is also used in medical machinery like MRI scanners, semiconductor manufacturing, cooling, and of course, meteorological balloons.
[ad_2]
Source link